|
| Gary Novak
The Cause of Ice Ages and Present Climate |
Heating cannot occur when 2,496 molecules around each CO2 molecule are unaffected by the process. A small amount of something can never heat a large amount of something without extreme differences in temperature. The extreme differences cannot exist in the atmosphere. The hotter anything gets, the faster it radiates away the energy. Carbon dioxide emits radiation much easier than it absorbs radiation, which keeps the temperature increase negligible. It emits as black body radiation (the wide bandwidth of total infrared radiation given off by the earth and all matter) while it absorbs fingerprint radiation, which is 8% of black body radiation. That's 12 times easier emitting than absorbing. Easier means one twelfth as much temperature increase by CO2 as cooling by the source. For every 1°C cooling at the source (such as total earth's surface), CO2 increases 1/12°C (0.08°C) (for total atmospheric CO2.)
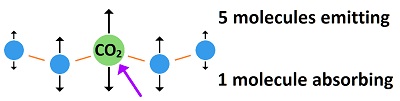
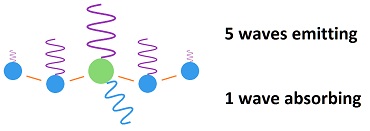
Carbon dioxide is a pass-through for energy, not an accumulator for energy. What this means is this: As a wave of fingerprint radiation (often 15 micron wavelength) hits a CO2 molecule, a molecular bond stretches within CO2. While that bond is stretching, the CO2 molecule is increasing its amplitude of emission. The energy leaves as fast as it is being absorbed with only one fourth being conducting to surrounding molecules. Temperature is an average vibration over many molecules. Each CO2 molecule would have to be about 2,500°C hotter than the atmosphere to create an average of 1°C increase for the whole atmosphere. But the needed 2,500°C is actually a fraction of a degree for the CO2. That's because there are 2,500 air molecules surrounding each CO2 molecule, which reduces the average that much. But one fourth of the energy would spread to surrounding molecules by conduction, which reduces the 2,500°C to 1875°C, which is just as ridiculous. It's 0.08°C for each 1°C cooling of the earth's surface due to radiation being emitted. Why one fourth? A CO2 molecule would lose about half of its gained energy in one half cycle as it bumped a nearby molecule (usually nitrogen). It would impart half of its excess energy into the molecule it bumps. One half of one half equals one fourth of the increased energy being imparted into the bumped molecule, for each wave of emitted radiation. This effect, caused by CO2 absorbing fingerprint radiation, rides over the top of heat entering the atmosphere through conduction, convection and evaporation and being radiated into space constantly. In other words, only a small part of the energy of CO2 is a result of absorption of fingerprint radiation. CO2 also picks up heat from the atmosphere through conduction. Climatologists don't view carbon dioxide as a pass-through for energy in the atmosphere, because they look at the spectrum for the whole atmosphere and see a dip for the main fingerprint frequency for carbon dioxide. What they miss is that while CO2 is absorbing fingerprint radiation it is emitting black body radiation. The emission disappears in the rest of the spectrum. Every molecule in existence absorbs and emits radiation constantly. Every molecule in existence absorbs and transfers heat by conduction constantly. Radiation and conduction create the second law of thermodynamics which says heat dissipates, always. Some scientists missed the fact that heat escapes so rapidly from the atmosphere that absorption by carbon dioxide becomes irrelevant. The added heat only sticks around for 83 femto seconds. That's not long enough to produce the slightest amount of heating. In fact, it is what all molecules do. Carbon dioxide is no different from any other molecule in the atmosphere for this. They all pick up heat and emit it through radiation combined with conduction. The atmosphere cools much more rapidly than the ground because of the high rate of emission of radiation from a transparent gas. While the air is cooling, it takes all heat equally. The heat of CO2 is no different from any other heat. A large part of the sun's energy gets into the atmosphere each day, and the same amount leaves each day. A miniscule effect by CO2 disappears in the process. Most heat gets into the atmosphere through conduction, convection and evaporation. Incompetent scientists didn't notice the conduction, convection and evaporation when they imagined a so-called greenhouse effect. When they later noticed the rest of the heat, they tried to fraction it into pieces and say some is due to a greenhouse effect. They had to use fraudulent math to get there. One of their major errors was in applying a false equation called the Stefan-Boltzmann constant and concluding that 79% of the energy leaving the surface of the earth is in the form of radiation. A white hot light bulb could not emit 79% radiation without a vacuum environment. The real amount would be 1-3%. There is so little energy in radiation at earth temperatures that the CO2 effect disappears with the rest of the radiation. Only with an absurdly high amount of radiation could a fraction of it be given a significance. If such a huge amount of radiation were leaving the earth's surface (79% of the energy), radiation would be leaving vastly more easily from the transparent gas of the atmosphere. At 40 times too much radiation given off by an opaque solid, the atmosphere would be losing heat much more than 40 times faster, and the temperature of the atmosphere would be extremely colder than it is. Here's a method of estimating the theoretical temperature increase of the atmosphere caused by carbon dioxide, which is 240 trillionths of a degree (240 x 10-12 °C): If the sun heats the Earth's surface 20°C every day, while cooling occurs that much each night, and radiation is 2% of the energy leaving the surface of the Earth, then cooling of the Earth's surface is 2% of 20°C, which is 0.4°C as radiation, while the other 19.6°C is due to conduction, convection and evaporation. This type of radiation is called black body radiation. It is the wide bandwidth of radiation emitted by all matter based on temperature. But CO2 in the air only absorbs 8% of the black body radiation as fingerprint radiation. So 8% of 0.4°C is 0.032°C as the temperature decrease at the surface of the Earth attributable to CO2 absorption. The 8% number was determined during the early 1950s before global warming became a social issue. Eight percent is approximately the proportion of the bandwidth attributable to CO2 absorption. This number does not evaluate total atmospheric dynamics but only the percent of the bandwidth available to CO2.
It means the 0.032°C cooling of the surface of the Earth will approximately equal 0.032°C heating of CO2 in the atmosphere. Each CO2 molecule in the air would have to be 2,500°C to average 1°C, since each CO2 is surrounded by 2,500 air molecules. But it reduces to 1875°C to produce an average temperature increase of 1°C for the entire atmosphere, after CO2 transfers one fourth of its energy by conduction to nearby molecules. A small amount of energy would be spread to nearby molcules before being radiated away. A CO2 molecule would lose about half of its gained energy in one half cycle as it bumped a nearby molecule (usually nitrogen). It would impart half of its excess energy into the molecule it bumps. One half of one half equals one fourth of the increased energy being imparted into the bumped molecule. The bumped molecule would do the same thing and impart one fourth of its added energy into the molecule which it bumps.
This means that three fourth of the energy picked up by CO2 is radiated away, while one fourth if added to nearby molecules. The energy cannot spread significantly before being radiated away by a small number of molecules. Dividing 1875°C by 0.032°C equals 58,594 times too little heating of the CO2 to increase the air temperature by 1°C average. Conservatives are saying they agree that some heating is due to a greenhouse effect, but it might be only half as much as claimed. Maybe it's 1/58,594 times as much as claimed. That's 0.000017 times as much as claimed. That's 17 millionths of a degree instead of 1°C heating upon doubling the amount of CO2 in the atmosphere. These temperatures are the amount of heating that would occur for each wave absorbed by CO2. With CO2 emitting radiation easier than it is absorbing, the heat is also lost with each wave being emitted. With one wave emitted while one wave is absorbed, the temperature increase would be reduced by a factor of about 0.14 times as much, which would be 2.4 millionths of a degree (0.0000024°C) instead of 17 millionths of a degree. Here's were the 0.14 comes from: The main bandwidth for CO2 fingerprint radiation is 15 microns, while the average emission for black body radiation is 25 microns. So waves are being absorbed 1.7 times faster than emitted (25 ÷ 17 = 1.7). This effect would increase heating of CO2 by a factor of 1.7. But the radiation absorbed by CO2 is only 8% of the black body bandwidth. So 8% of 1.7 equals 0.14. This result would occur if there were no such thing as saturation. Saturation means all radiation available is absorbed by a tiny amount of CO2 in the air, so more CO2 cannot absorb more radiation. Heinz Hug did a laboratory measurement and said all radiation is absorbed by CO2 as it travels 10 meters near the Earth's surface. That means nothing more happens when CO2 is doubled in the atmosphere besides reducing the 10 meters to 5 meters, which is irrelevant.
This means the shoulders of the absorption curve represent CO2 molecules in a different energy state due to bonds vibrating. There are very few of these CO2 molecules, so radiation must travel farther before being completely absorbed at shoulder wavelengths. But to not saturate in the troposphere would mean there are extremely few. Most shoulder molecules do saturate in the troposphere. One thousandths of the CO2 molecules would absorb in 10 kilometers instead of 10 meters, which is still in the troposphere. So divide the result by about one ten thousandths for actual absorption increase. Then, 2.4 millionths of a degree becomes 240 trillionths of a degree. The atmosphere heats 240 x 10-12 °C upon doubling the amount of CO2 in the atmosphere. This number is the hypothetical potential of CO2 to heat the atmosphere. In actuality, water vapor absorbs most of the same radiation due to overlap of absorption spectra, so there is even less heating due to CO2 under atmospheric conditions.
In 2001, the IPCC (AR3) stated that saturation exists in these terms:
With this statement there was no explanation of how the shoulder molecules are evaluated for the magical heating, and there was apparently no further mention of shoulder molecules or saturation in subsequent publications of the IPCC reports. There is a naive concept that everything is always the same, so something different must be a disturbance of nature. Nothing resembling it is reality. Weather, climate and geological history are off the graphs for earth shaking effects. An ice age occurs every 100,000 years. That's an instant in geological time and human history. We are constantly undergoing change as a result. And while that is happening, everything is in constant turmoil. A "Medieval Warm Period" and "Little Ice Age" occurred in recent history. Solar energy appears to be constantly increasing and decreasing, but it is about impossible to measure the slow variations. Solar energy accumulates in the oceans, which appears to be the cause of a mysterious temperature increase in the oceans at this time. But more recently, we are told that solar energy is decreasing with an absence of sun spots, which could indicate another small ice age is coming. Air does not hold enough heat to transfer the slightest amount of heat to the oceans. Incompetent scientists never account for the heat capacity. The Math Of Heat Capacity Here's an analogy. A chemist is buying a new house. It has plaster in the walls consisting of calcium sulfate. But the chemist determines that one part per thousand is calcium chloride instead of calcium sulfate. Calcium chloride absorbs radiation at a slightly different wavelength than calcium sulfate. So he refuses to buy the house, because it will be harder to heat than it would be if the plaster were all calcium sulfate. And to be convincing, he lies about it and says the air will have to be 2°C hotter to heat the house. The Nonsense Of Radiative Transfer Equations
|


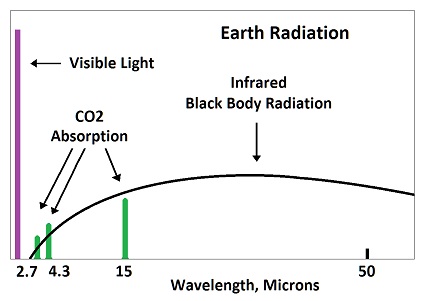
 The dip tells them that CO2 is absorbing radiation.
The dip tells them that CO2 is absorbing radiation.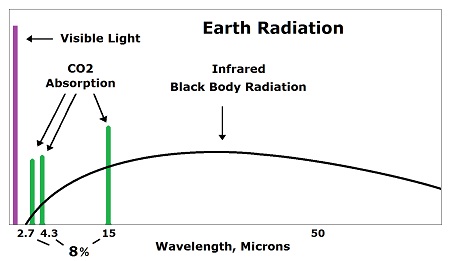
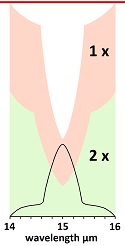 Climatologists admit that saturation occurs, but they claim only the center of the absorption peak saturates, while the shoulders do not saturate.
Climatologists admit that saturation occurs, but they claim only the center of the absorption peak saturates, while the shoulders do not saturate.